REAL-WORLD EXAMPLE: STREAMLINED OPERATIONS, KEEPING PATIENTS SAFE AND STOPPING FAKE DRUGS
Better Tracking and Monitoring
Galderma, a global dermatology leader, proactively implemented serialization and aggregation solutions beyond regulatory compliance. Partnering with OPTEL, the company integrated automated systems to assign unique serial numbers and create a “parent-child” relationship between packaging levels. This enhanced visibility across production, improved product reconciliation, and optimized warehouse operations, including recalls and reverse logistics. By anticipating future regulations, Galderma streamlined compliance while positioning itself for smarter supply chain management. The move not only ensured accuracy in tracking but also reinforced brand trust through transparency, meeting growing consumer demands for authenticity and quality assurance.
A top global pharmaceutical company in Japan needed serialization and aggregation solutions for its Netherlands-based lines to comply with international regulations. Facing high-speed production (100–300 CPM), space constraints, and a tight six-month deadline, the company sought an efficient, cost-effective solution. OPTEL successfully integrated six InspectProof™ systems into existing cartoners, enabling seamless serialization without disrupting operations. Additionally, BundleTracker™ and PackStation™ SAP solutions were deployed to facilitate aggregation and supply chain visibility. OPTEL’s flexible, tailored approach ensured compliance while optimizing costs and operational efficiency.
Rapid Recalls
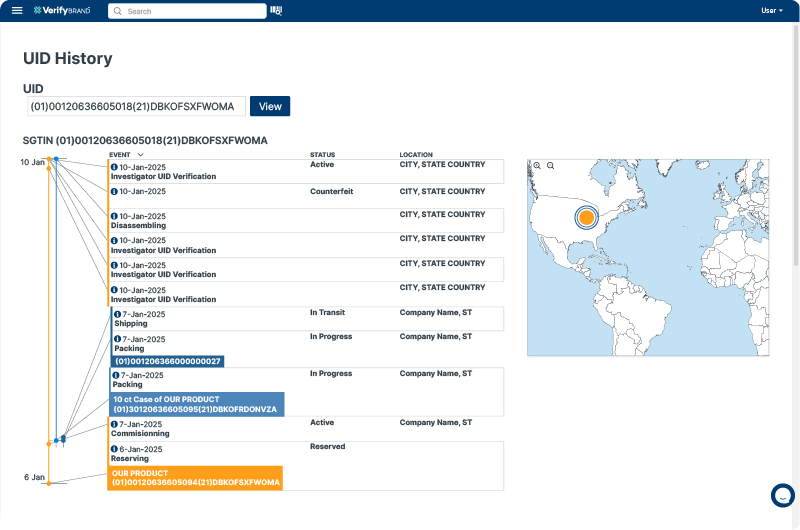
In cases of quality issues or required product recalls, aggregation technology enables rapid, targeted responses. By tracing the hierarchical relationships in packaging units, companies can promptly identify and recall specific units or batches, thus minimizing patient impact and risk of adverse outcomes.